Many scientists and researchers are eager to develop a vaccine for COVID-19, a disease that has, to date, affected millions of people worldwide. Unfortunately, there is currently no vaccine available to the general public. However, there are alternative remedies that people are turning to in order to prevent or cure COVID-19, such as convalescent plasma. Patients who have fully recovered from COVID-19 can donate their blood or plasma to help those who are currently sick with the disease.
What is convalescent plasma therapy?
On August 23, 2020, the Food and Drug Administration (FDA) re-authorized the use of convalescent plasma for treating severe COVID-19 cases. Convalescent plasma is donated from patients who have recently been infected and recovered from COVID-19. Plasma is the clear, straw-colored portion of blood that contains COVID-19 antibodies. Antibodies are generated by the immune system to fight against diseases. Doctors can use the donated plasma of recovered patients and use it treat people who are currently infected. The use of convalescent plasma is not a new phenomenon. In fact, convalescent plasma has helped patients recover from other serious diseases, such as rabies, hepatitis B, polio, measles, influenza, and Ebola.
Previously, the FDA had authorized the emergency use IND (eIND) back in March, which allowed healthcare professionals to treat patients with COVID-19 through convalescent plasma donations. Additionally, the FDA also approved the National Expanded Access Protocol on April 7, 2020. This allowed a wider range of subjects to be tested, from people who are at risk of severe disease and people who are still suffering from severe disease. After promising preliminary results from nationwide studies, the FDA has reauthorized the emergency use of convalescent plasma. Convalescent plasma now joins remdesivir, a broad spectrum antiviral medication from Gilead Sciences Inc. as the only two FDA authorized treatments for COVID-19.
What research has been done on convalescent plasma?
Several research trials have shown progressive results for the use of convalescent plasma for COVID-19 treatment. The FDA’s action was primarily based on recent data from a large multi-centric study led by Mayo Clinic in Rochester, Minnesota. Their study of 72,000 patients reported that patients treated with higher levels of antibodies from convalescent plasma had a higher chance of survival compared to those treated with lower level of antibodies. Another study from Houston Methodist of 300 patients found out that patients treated with high antibody plasma within 72 hours of hospitalization were more likely to survive than patients who did not receive any plasma.
Most patients in these trials fully recovered from COVID-19, and early data shows that convalescent plasma may be a promising treatment. However, the studies do have limitations on evaluating the efficacy of treatment response due to multiple factors. COVID-19 subjects may also be receiving treatment of antibiotics, antivirals, antifungals, and corticosteroids at the same time, which all affect treatment outcomes. At this time, studies suggest that plasma may lessen the severity or shorten the duration of COVID-19. Whether plasma is an effective treatment or cure for COVID is unknown and further studies are needed. For now, convalescent plasma may be a potential bridge for physicians to treat COVID patients while other therapies and potential vaccines are under development.
Who can donate convalescent plasma?
For those wanting to sign up to donate convalescent plasma, there are specific criteria that need to be met. Donors should have been tested positive for COVID-19 or have lab evidence of existing SARS-CoV-2 antibodies. Donors should have recovered from COVID-19 or be symptom-free for at least fourteen days. A negative test for active COVID-19 disease is not required to donate. Lastly, donors must pass the standard eligibility requirements to be a blood donor.
I haven’t had COVID-19. Is there anything I can do to help?
For those who have not had COVID-19, you can help by donating blood! The COVID-19 pandemic with its implementation of physical distancing has led to the cancellation of many blood drives across America. This has led to a shortage of the national blood supply, which is important for many life-saving procedures such as trauma emergencies and surgeries. You can find local blood donor centers around you to schedule your donation online via American Association of Blood Banks (AABB), American Red Cross, America’s Blood Centers, and more.
What are the risks of donating and receiving plasma?
Donating plasma is generally very safe. The risks of plasma donation in a healthy donor are minimal but may include dehydration, dizziness, fainting, and lightheadedness.
Before you go right here and get assistance for treatment, there are things to know. For COVID-19 patients being treated with donated plasma, there are possible risks that could occur just like with any other plasma or blood transfusion. These possible risks include allergic reactions, infections (such as HIV, Hepatitis B or C), transfusion-associated circulatory overload (TACO), transfusion-associated acute lung injury (TRALI), or possible aggravation of immune-mediated tissue damage by ADE (antibody-dependent enhancement). The most common cause of transfusion related fatality is TRALI, where the patient receiving the donated plasma has lung damage and difficulty breathing.
Fortunately, there have been no documented issues arising with antibody-dependent enhancement through convalescent plasma donations. There has been no noted transmission through blood of the SARS-CoV-2 virus, so any transfusion associated problem from a recovered donor is extremely unlikely. However, the outcomes outweigh the risks as these trials of convalescent plasma donations may help ward off the virus from those affected.
How does convalescent plasma donation work?
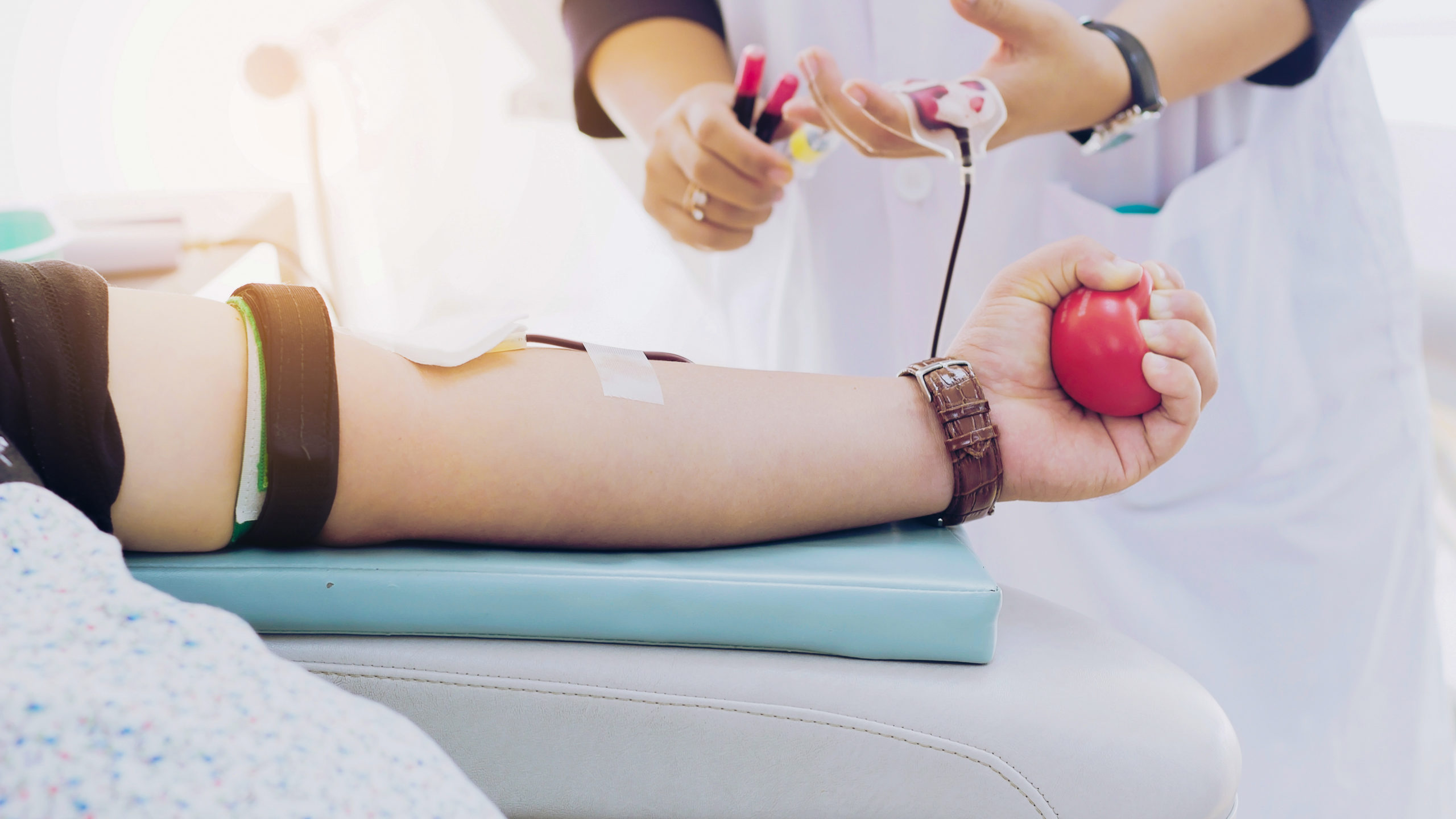
Convalescent plasma donations are collected through the blood donation process. The donation process can last from one to two hours long. The donation of plasma is specific to one’s blood type, so it is important to classify the plasma as being type A, B, O, or AB. The best type of plasma to have is AB because it is a universal plasma. This means that AB plasma can be transfused to all patients, no matter what blood type they are. A machine will draw all blood, separate the plasma, and then return the rest of the blood back to the donor.
With healthcare workers trying to fight against the spread of COVID-19, many are hopeful that convalescent plasma will help people infected by COVID-19. Convalescent plasma treatment results have been promising from research institutes, such as the Mayo Clinic and the Michigan State University.
For those who have recovered from COVID-19, please consider donating convalescent plasma as it can be lifesaving for those who are fighting the disease. Your single donation can treat up to three patients with COVID-19.To find out more about blood donations, please review the donation requirements from the American Association of Blood Banks.